Key points:
— Gene drive is a type of genetic engineering technique. It permits the modification of genes so they do not follow the regular rules of heredity. The method can be used to reduce certain qualities in species, for the purpose of extermination or prolonging a lifeline.
— More than 200 organisations worldwide are calling for global cooperation and decision on the release of gene drive organisms to protect biodiversity. Despite that, there is significant resistance against the technology coming from different stakeholders and the public.
— Overall, it seems that the benefits of rigorous legislative intervention, in the form of a convention rather than a guidebook, could be a more consensus-building and effective option.
Introduction
‘Should humanity release genetically engineered gene drive organisms into nature?’
This was a question asked by several NGOs in 8 European countries — Bulgaria, Denmark, Germany, France, Italy, Sweden, Spain, and Poland. As reported, the most popular answer was: ‘No, the risks are too high’. The survey considered 9,000 people, to be representative of 280 million EU citizens[1].
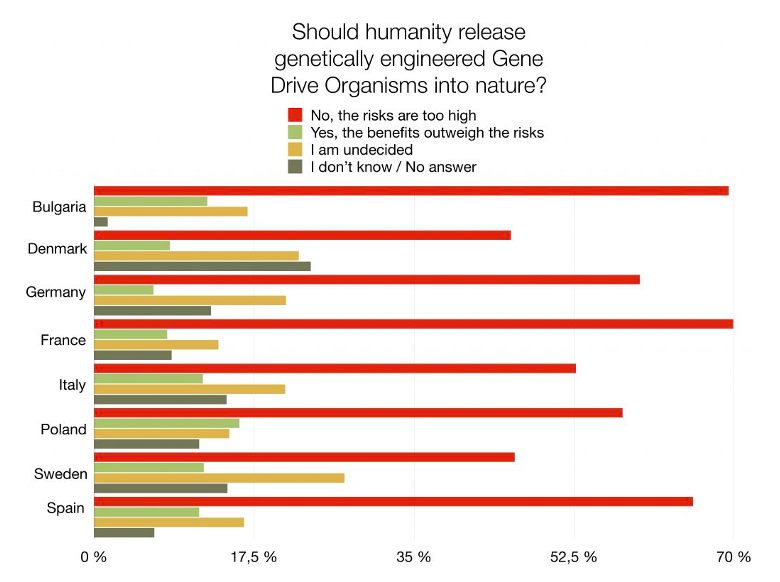
The answer – surprising or not – illustrates the general fear of the new which may, or may not, harm biodiversity, human health, agriculture, or world peace[2]. The consensus is situated on the notion that only when the consequences of releasing gene drive animals into the wild are fully explored, will the introduction be, potentially, acceptable. Nevertheless, this line of thinking brings forward the chicken-egg problem — without introducing such organisms into the environment, it might be difficult or impossible to investigate, with certainty, the consequences of the freeing. The regulation of biotechnology is usually troublesome. In science, uncertainty is always involved[3] and probabilities in risk assessment are never 0 or 100%[4].
More than 200 organisations worldwide are calling for global cooperation and decision on the release of gene drive organisms (GDOs) to protect biodiversity[5]. A step forward, one that could also appease the opposers of the idea, is to create a set of rules and regulations which could aid the supervision of the process and ensure control against the unwanted overexpansion. In the absence of such, scientists decide to create their own guidelines, voluntarily. This, unfortunately, renders to be insufficient to allow wide implementation of the technology and the natural habitat testing[6]. Hence, what these hard law regulations[7] could look like and how the general breeding of the animals, as preceding the release, should be regulated will be the subject of this article. This work is to serve as a general overview of the matter, providing some ground points for legal consideration.
Gene drive organisms — definition, advantages and disadvantages
Gene drive is a type of genetic engineering technique. It permits the modification of genes so that they do not follow the regular rules of heredity[8]. It is a new development, which even two years ago was doubted to come about[9]. The method can be used to reduce a certain quality in species, for the purpose of extermination or prolonging of a lifeline.
Importantly, such organisms also fall under the category of genetically modified organisms (GMOs). What comes as a new factor, however, is that this gene modification can be inherited, at least in most cases, ensuring expansion of the improved genome. This can be the panacea for areas prone to incubating diseases, with invasive species, agricultural pests, and predators of endangered species. It could also ensure that the mutation of viruses is not possible in some species. This shields from pandemics and makes the environmental and biodiversity efforts more efficient and effective[10]. A prime example thereof is the control of mosquito populations to prevent the spread of malaria[11].
Indeed, the technology is of special interest to Africa, with manifold African organisations taking an active part in research and discussions on the introduction and regulation of GDOs[12]. The reason for such interest is also the cost- and labour-saving aspect of the technology, if implemented on a wide scale[13]. Interestingly, gene drives are said to have a lesser negative impact on the environment than the traditional ways of species regulation[14]. This is why the technology is also important for Australian efforts to protect native animals from feral cats[15].
The risks of such genetic interference are, as said, widely unknown. Its opponents speak of the uncontrollability of future processes, including the cross-breeding of gene drive and natural organisms. There are also factors, such as contamination with GMOs, that could potentially have catastrophic effects on the gene drive population and others, consequently destroying the biodiversity and habitats as we know them[16]. And the reversal in such a case might be impossible, posing additional challenges and regulatory problems, especially if the modified individuals manage to cross borders[17]. Exactly that last aspect is also what calls for uniform global action, as has been highlighted by scholars coming from seemingly contained environments, such as New Zealand[18]. It is also worth noting that some states are not capable of developing a comprehensive regulatory scheme for gene drives by themselves, hence, international aid (in the form of cross-border study group help or international laws integrated into national systems) would be in some cases simply necessary[19].
Risk for human gene driving
An important matter to remark on, to make the discussion more complete, is the fact that gene drives could be developed and applied for any species that reproduces sexually, including humans. Hence, there might be some questions regarding the plausibility of the risk that one will develop ‘advanced’ humans, in the spirit of transhumanism, if the technology is more widely regulated and hence implemented. Albeit the risk exists, naturally, the degree of plausibility is low. The technology is beneficial for species that reproduce quickly, which, as compared to mosquitoes, for example, is not accurate for humans. The generation time for mosquitoes is around four weeks, in contrast to human 20-30 years; therefore, a gene drive could change the genetics of a mosquito population within a few years, not decades[20]. The interest in such a modification enterprise for humans, and the possibility of benefiting from such, if ever somewhat accepted even by the most progressive, is unlikely.
Legal response
The debate on the ethicality, scientific and societal necessity is one matter. The discussion on how to permit such processes of creation and introduction is another. What is crucial to note, however, is that appropriate engagement with stakeholders is essential, taking the potential ethical doubts concerning the use of the technology, as relevant for the more and more affluent principles of animal rights[21]. Neglect of such action could lead to further procedural problems and instability of the built framework. To restate, a global, common approach ought to be sincerely considered.
A crucial development at this stage is the US National Institutes of Health (NIH)’s focus on highlighting the importance of including the community engagement efforts and opening communication among stakeholders, communities, and the public within the governing framework[22].
The creation
Some states, for example, Mexico, introduced bills dedicated solely to the creation of GDOs; however, such regulations are not common[23]. Since GDOs fall under GMOs, they are ordinarily, in the absence of lex specialis, regulated under manifold national and international laws governing such.
Despite the possible incentive to also propose specific rules on the creation of GDOs, there seems to be no pressing need for such an action. Typically, nowadays, research with novel GDOs must take place in laboratories, as is the case of any other new type of GMO. Mostly, the laboratories that work with the technology obtain permits similar to the ones granted for GMOs. At times, the only requirement making a difference in the matter is the need for upgrading research facilities to meet new containment requirements, training staff on new protocols, and undergoing additional national inspections[24]. Such regulations could be considered internationally, yet it seems that most countries manage to successfully deal with the peculiarities under the GMO laws.
The introduction
No state has allowed researching GDOs in natural habitats, and no case of release of an organism with a gene drive has been officially reported[25].
The experiences with related technologies have been suggested as an alternative, especially if those share some properties with gene drives techniques; examples being: the release of biological control agents, sterile insect technique, transgenic insects, or Wolbachia-based gene drive organisms[26]. All in all, this is not seen as a sufficient replacement, or one that would provide any valuable input; hence, field trials are still considered inevitable[27]. National Environmental Agencies, in the aftermath of the discussions on the EU level by the European Commission and the European Food Safety Authority (EFSA) in 2018, established that national regulations and Environmental Risk Assessments (ERA) will not suffice to cover all the possible health and environmental risks that GDOs might bring[28]. Of relevance for the GDOs release is to see them spread in the environment — to check their influence. The existing regulations pertain mostly to GMOs and require a step-by-step approach of a slow introduction in isolated environments — which is of no far-reaching use for the GDO problem matter[29].
Another alternative is the creation of artificial environments, mimicking the natural habitats perfectly. This has been successfully arranged for gene drive mosquitoes[30]. Naturally, however, this might not be viable for other species; and the idea is still taken with a grain of salt by many scholars[31].
The introduction — case-based approach
In India, building on the existing case-on-case regulatory approach, the Takshashila Institution, a public policy school and think tank, provided a risk assessment report on the testing and introduction of gene drives. There are three assessment stages, proposed with the focus on risk assessment and potential mitigations. The research done by the institution provides useful insights on the governance of gene drives, for all applications of the technique, across all planes[32].
In such spirit, the design of post-introduction monitoring must be science-based. Drawing from examples such as of the Takshashila Institution, some insist on a wider, case-by-case approach to each specific type of GDOs[33]. This, naturally, does not perpetuate the idea of creating a consistent framework and procedure that could be easily replicated each time — one that would take away the additional costs of money and time, needed for appropriate assessment of each case. Yet, this alternative would also allow for more certainty regarding safety, as each type of organism would be thoroughly examined for each specific environment, etc. What could appear are legal handbooks, guides, that one could check off while assessing each specific case, if indeed scientific procedure was to be appropriately followed. There are some guidelines in place for mosquito testing, issued by the WHO; nonetheless, their soft-law and general character (not to mention that they do not include explicit public oversight and implementation requirements) have been deemed grandly insufficient in their contemporary form[34].
Taking the aforementioned contributions, such guidelines could include case-specific stage-by-stage recommendations, involving the advice on the frequency of intervention, markers of progress or its lack, the quality and presence of halting factors, and the time which would be appropriate for a trial duration[35]. As said, this would have to be consulted with the scientific discoveries as of the most recent, and ideally updated with each new major development of the technology. Open access and transparency requirements are key.
The introduction — (more) universal approach
Another option would be to develop a uniform framework of rules which guide the introduction of all types of gene drives. This is Occam’s razor solution, with its merits and drawbacks. The major benefit is undoubtedly the utility or universalism of the choice, making the procedure more replicable, and, importantly, traceable top-down.
Nevertheless, it presents several challenges. Undoubtedly, such a document would have to be lengthy and very precisely drafted, for all possible events, just to ensure due care for safety. Regular amendments would be expected, to ensure the problems brought by new developments in the speedily advancing technological field are appropriately addressed. Yet, despite the seeming hurdles, this does not have to be deemed prohibitive if one considers that the alternative is to create specific guidelines for each species or each environment, as discussed above. Certainly, the proposed contents of the specific guidelines can be adopted to this alternative. Further, if it is only possible, general and all-encompassing provisions would be advised; however, taking the complexity of the phenomenon, the potency of these organisms to affect the environment to an unknown degree, there is a chance such type of provisions would not be recommended by the scientific sector, and that it would not appease the public, taking its vociferous contempt, as expressed in the past[36].
Introduction — soft or hard law
The points raised for both options ought to be considered to find the best, feasible solution to the international problem. Nevertheless, it is paramount to consider what type the laws should be — soft or hard, whether these should be recommendations or strict rules placed in a convention or a treaty.
As mentioned above, the already-developed, voluntary networks were not satisfactory. The reason for this could lie in their ad hoc character and the fact that they are drafted individually, without broader consultations. But setting suggestions over strict rules could facilitate the introduction of transitional phases[37] and prevent the setting of unachievable standards, especially for developing countries. This, as one may quickly remark upon, does not answer the concerns of safety. If global cooperation in a standardised manner is expected, non-binding rules might become neglectable and redundant the minute they are introduced; and replaced by a binding convention.
Since the problem is multinational, set rules included in a convention would be the more adequate option. Uniformity might be the solution ensuring certainty, on the plethora of concerned levels. This option solves the problem raised for the soft law guidelines. Naturally, hard law is at times inflexible and might create additional hurdles to its subjects. Nonetheless, when speaking of a technology that might be dangerous if unsupervised in an adequate manner, the problems of inhibiting some users’ entrance to the market may be disregarded. Concerning the inflexibility, this can be significantly amended if the law is drafted with care and its contents revisited if the situation requires.
Notably, there seems to be a preference for conventions. For example, the paramount existing document in the matter, for GMOs in general, is the United Nations Convention on Biological Diversity, as implemented through the Cartagena and Nagoya Protocols. Many states have considered this regulation while drafting their own laws, inheriting its inhibiting approach towards the development of the technology. Crucially, some key powers, such as the United States, are not part of the protocol, creating another possible discrepancy in the development of a uniform approach. This, however, might be inevitable when any legal document is considered on the international level — voluntarily being the often condemned, yet intrinsic feature of international law. Again, this might be avoided if the law is well-constructed, in consultation with its signees, as well as science and the public.
In the European Union, the central law regulating the release is to be found in the directive regarding GMOs, Directive 2001/18/EC, also known as the ‘Release Directive’, one of the most comprehensive and useful documents for the matter at hand. The Directive could be a sample for a singular regulation on GDOs. The EU document presents a common methodology to assess risks for the environment and defines the common objectives for the monitoring of the released GMOs. It also sets technical requirements for the trials.
As a technical side-note, the EU document includes a mechanism that modifies, suspends, or terminates the released organisms if they render dangerous for one reason or another. This, however, might be challenging for gene drives, as their reproduction is hard to monitor and prevent if the organism is freely released to naturally interact in the environment. This is one aspect the sought convention should consider.
Summary
Overall, it seems that the benefits of rigorous legislative intervention, in the form of a hard law convention rather than a guidebook could be a more consensus-building and effective option. The choice between an all-encompassing or case-based approach is difficult to make; however, taking the chosen form of the law, it might be that the former option must be adopted, for the sake of reachability and obtaining a consensus on the international level. Naturally, a middle option could be developed, in the form of a law that considers the most prominent cases in detail and handles the specific cases in a general manner.
The matter raises much controversy, simply because of the involved uncertainty and somewhat abstractness of the problem. This response might also indicate that the possible advantages of the technology are noticed, and the possible inevitability of the use is acknowledged. What is at stake is finding a way to reduce the troubles of one of the most disadvantaged continents, Africa, or, if investigated further, possibly preventing the development of animal-based viruses. Especially the public health aspect ought to be a sufficiently pressing incentive to start considering the GDO laws more intensively and drafting regulations that could prevent an unnecessary halting of the implementation. Yet, as expressed before[38], an adequate approach to the development and governance of GDOs must go beyond considerations for public health and the environment and also consider the benefits of technological innovation, the implications of intellectual property, public engagement, and economics. Law as a discipline tends to be slow at catching up to the changes the world experiences. This has had detrimental consequences in the past. Technology law as a discipline must react swiftly to be considered apt and capable.
[1] 2021. “Survey: EU citizens reject genetic engineering of wild species with Gene Drives”. Stop Gene Drives. https://www.stop-genedrives.eu/survey-eu-citizens-reject-genetic-engineering-of-wild-species-with-gene-drives/ accessed 7 September 2021.
[2] 2021. “Survey: EU citizens reject genetic engineering of wild species with Gene Drives”. Stop Gene Drives. https://www.stop-genedrives.eu/survey-eu-citizens-reject-genetic-engineering-of-wild-species-with-gene-drives/ accessed 7 September 2021.
[3] Tait, J. 2014. “Bringing it all together”. Innovation: Managing Risk not Avoiding It. Evidence and Case Studies, Annual Report of the Government Chief Scientific Adviser. https://www.gov.uk/government/publications/innovation-managing-risk-not-avoiding-it accessed 11 September 2021.
[4] Charo, A. 2015. “Comparative approaches to biotechnology regulation. Presentation at the International Summit on Human Gene Editing. December 1-3, 2015. Washington, D.C”. Vimeo. https://vimeo.com/album/3703972/video/149182567 accessed 11 September 2021.
[5] “Moratorium on Gene Drive Organisms”. We Move Europe. https://act.wemove.eu/campaigns/gene-drive-moratorium-INT-EN accessed 8 September 2021.
[6] “Gene-drive technology needs thorough scrutiny”. Nature. https://www.nature.com/articles/d41586-017-08214-4 accessed 10 September.
[7] Binding law in contrast to non-binding, so-called soft law.
[8] Coffey D. 2020. “What is a gene drive?”. Live Science. https://www.livescience.com/gene-drive.html accessed 8 September 2021.
[9] Scudellari M. 2019. “Self-destructing mosquitoes and sterilized rodents: the promise of gene drives”. Nature. https://www.nature.com/articles/d41586-019-02087-5 accessed 8 September 2021.
[10] “Gene drives: benefits, risks, and possible applications”. Swiss Academy of Science. https://api.swiss-academies.ch/site/assets/files/5105/factsheet_gene_drives_e_online.pdf accessed 8 September 2021.
[11] Scudellari M. 2019. “Self-destructing mosquitoes and sterilized rodents: the promise of gene drives”. Nature. https://www.nature.com/articles/d41586-019-02087-5 accessed 8 September 2021.
[12] Kelsey A et al. 2020. “Global Governing Bodies: A Pathway for Gene Drive Governance for Vector Mosquito Control”. Am J Trop Med Hyg. 2020 Sep; 103(3): 976–985. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7470596/ accessed 8 September 2021.
[13] Harvey-Samuel, T., Ant, T. & Alphey, L. 2017. “Towards the genetic control of invasive species”. Biological invasions 19 (6): 1683–1703.
[14] Campbell, K. et al. 2015. “The next generation of rodent eradications: innovative technologies and tools to improve species specificity and increase their feasibility on islands”. Biol. Cons. 185: 47–58.
[15] Kachel N. 2018. “Gene drive technology: A new hope in the fight against feral cats”. CSIROscope. https://blog.csiro.au/gene-drive-technology-a-new-hope-in-the-fight-against-feral-cats/ accessed 10 September 2021.
[16] “Gene Drive Organisms: Destructive and uncontrollable”. Swissaid. https://www.swissaid.ch/en/articles/gene-drives/ accessed 8 September 2021.
[17] “Gene drives: benefits, risks, and possible applications”. Swiss Academy of Science. https://api.swiss-academies.ch/site/assets/files/5105/factsheet_gene_drives_e_online.pdf accessed 8 September 2021.
[18] Terry S., Howard S. 2018. “A Constitutional Moment – Gene Drive and International Governance”. NZLFRRp 4. http://www.nzlii.org/nz/journals/NZLFRRp/2018/4.html accessed 10 September 2021.
[19] Committee on Gene Drive Research in Non-Human Organisms: Recommendations for Responsible Conduct; Board on Life Sciences; Division on Earth and Life Studies; National Academies of Sciences, Engineering, and Medicine. 2016. “Gene Drives on the Horizon: Advancing Science, Navigating Uncertainty, and Aligning Research with Public Values”. National Academies Press (US). https://www.ncbi.nlm.nih.gov/books/NBK379267/ accessed 10 September 2021.
[20] “Gene drive research: why it matters”. https://royalsociety.org/~/media/policy/Publications/2018/08-11-18-gene-drive-statement.pdf accessed 10 September 2021.
[21] Another article of the author relating to the (un)ethicality of some practices with the use of animals was published by Instytut Nowej Europy here: https://ine.org.pl/en/a-short-introduction-to-patenting-of-animals-and-a-discussion-on-the-legal-morality-of-such-2/.
[22] Kelsey A et al. 2020. “Global Governing Bodies: A Pathway for Gene Drive Governance for Vector Mosquito Control”. Am J Trop Med Hyg. 2020 Sep; 103(3): 976–985. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7470596/ accessed 8 September 2021.
[23] “Human and Agriculture Gene Editing: Regulations and Index“. Global Gene Editing Regulation Tracker. https://crispr-gene-editing-regs-tracker.geneticliteracyproject.org accessed 10 September 2021.
[24] “Facts about Gene Drive: Regulating Gene Drive Research”. Outreach Network for Gene Drive Research. https://genedrivenetwork.org/resources/factsheets/8-factsheet-how-is-gene-drive-research-regulated-july2018/file accessed 10 September 2021.
[25] For example: “Gene drives: benefits, risks, and possible applications”. Swiss Academy of Science. https://api.swiss-academies.ch/site/assets/files/5105/factsheet_gene_drives_e_online.pdf accessed 8 September 2021; 2017. “Gene-drive technology needs thorough scrutiny”. Nature. https://www.nature.com/articles/d41586-017-08214-4 accessed 10 September; “Human and Agriculture Gene Editing: Regulations and Index“. Global Gene Editing Regulation Tracker. https://crispr-gene-editing-regs-tracker.geneticliteracyproject.org accessed 10 September 2021.
[26] “Gene drives: benefits, risks, and possible applications”. Swiss Academy of Science. https://api.swiss-academies.ch/site/assets/files/5105/factsheet_gene_drives_e_online.pdf accessed 8 September 2021.
[27] “Gene drives: benefits, risks, and possible applications”. Swiss Academy of Science. https://api.swiss-academies.ch/site/assets/files/5105/factsheet_gene_drives_e_online.pdf accessed 8 September 2021.
[28] EPA/ENCA Interest Group on Risk Assessment and Monitoring of GMOs. 2019. “Gene Drive Organisms: Implications for the Environment and Nature Conservation”. Report REP-0705. https://www.umweltbundesamt.at/fileadmin/site/publikationen/rep0705.pdf accessed 9 September 2021.
[29] National Academies of Sciences, Engineering, and Medicine, Division on Earth and Life Studies, Board on Life Sciences, Committee on Gene Drive Research in Non-Human Organisms: Recommendations for Responsible Conduct. “Gene Drives on the Horizon: Advancing Science, Navigating Uncertainty, and Aligning Research with Public Values”. 2016. National Academies of Sciences, Engineering, and Medicine. https://books.google.lt/books?hl=en&lr=&id=pLqhDQAAQBAJ&oi=fnd&pg=PR1&dq=(2016)+Gene+Drives+on+the+Horizon:+Advancing+Science,+Navigating+Uncertainty,+and+Aligning+Research+with+Public+Values+(National+Academies,+Washington,+DC).&ots=bSIjdSmc6c&sig=9su2WTtXQU5Dpq-f-Xj1T-zR51c&redir_esc=y#v=onepage&q=(2016)%20Gene%20Drives%20on%20the%20Horizon%3A%20Advancing%20Science%2C%20Navigating%20Uncertainty%2C%20and%20Aligning%20Research%20with%20Public%20Values%20(National%20Academies%2C%20Washington%2C%20DC).&f=false accessed 8 September 2021.
[30] “Human and Agriculture Gene Editing: Regulations and Index“. Global Gene Editing Regulation Tracker.
[31] Committee on Gene Drive Research in Non-Human Organisms: Recommendations for Responsible Conduct; Board on Life Sciences; Division on Earth and Life Studies; National Academies of Sciences, Engineering, and Medicine. 2016. “Gene Drives on the Horizon: Advancing Science, Navigating Uncertainty, and Aligning Research with Public Values”. National Academies Press (US). https://www.ncbi.nlm.nih.gov/books/NBK379267/ accessed 10 September 2021.
[32] Kelsey A et al. 2020. “Global Governing Bodies: A Pathway for Gene Drive Governance for Vector Mosquito Control”. Am J Trop Med Hyg. 2020 Sep; 103(3): 976–985. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7470596/ accessed 8 September 2021.
[33] EPA/ENCA Interest Group on Risk Assessment and Monitoring of GMOs. 2019. “Gene Drive Organisms: Implications for the Environment and Nature Conservation”. Report REP-0705. https://www.umweltbundesamt.at/fileadmin/site/publikationen/rep0705.pdf accessed 9 September 2021.
[34] Committee on Gene Drive Research in Non-Human Organisms: Recommendations for Responsible Conduct; Board on Life Sciences; Division on Earth and Life Studies; National Academies of Sciences, Engineering, and Medicine. 2016. “Gene Drives on the Horizon: Advancing Science, Navigating Uncertainty, and Aligning Research with Public Values”. National Academies Press (US). https://www.ncbi.nlm.nih.gov/books/NBK379267/ accessed 10 September 2021.
[35] Some of these have been already recommended by the scientific sector, for example, in recommendations by: Committee on Gene Drive Research in Non-Human Organisms: Recommendations for Responsible Conduct; Board on Life Sciences; Division on Earth and Life Studies; National Academies of Sciences, Engineering, and Medicine. 2016. “Gene Drives on the Horizon: Advancing Science, Navigating Uncertainty, and Aligning Research with Public Values”. National Academies Press (US). https://www.ncbi.nlm.nih.gov/books/NBK379267/ accessed 10 September 2021.
[36] Including some institutions such as the Royal Society calling the UN not to approve of trials: “Gene drive research: why it matters”. https://royalsociety.org/~/media/policy/Publications/2018/08-11-18-gene-drive-statement.pdf accessed 10 September 2021.
[37] Synthetic Biology Leadership Council. 2016. Biodesign for the Bioeconomy: UK Synthetic Biology Strategic Plan 2016. https://connect.innovateuk.org/documents/2826135/31405930/BioDesign+for+the+Bioeconomy+2016+-+DIGITAL.pdf/0a4feff9-c359-40a2-bc93-b653c21c1586 accessed 11 September 2021.
[38] Committee on Gene Drive Research in Non-Human Organisms: Recommendations for Responsible Conduct; Board on Life Sciences; Division on Earth and Life Studies; National Academies of Sciences, Engineering, and Medicine. 2016. “Gene Drives on the Horizon: Advancing Science, Navigating Uncertainty, and Aligning Research with Public Values”. National Academies Press (US). https://www.ncbi.nlm.nih.gov/books/NBK379267/ accessed 10 September 2021.
IF YOU VALUE THE INSTITUTE OF NEW EUROPE’S WORK, BECOME ONE OF ITS DONORS!
Funds received will allow us to finance further publications.
You can contribute by making donations to INE’s bank account:
95 2530 0008 2090 1053 7214 0001
with the following payment title: „darowizna na cele statutowe”
































Comments are closed.